Our Research
Sustainability through Catalysis and Continuous Flow Platforms
Our research is based on the design and development of new and better heterogeneous catalysts by investigating the environment of the catalyst active site during the reaction at the molecular level using conventional ex situ and special in situ techniques developed at Queen’s University Belfast. We study the interactions between active metal species, the surrounding solvent molecules and surface adsorbed molecules (solvent, reactants and products). This information is critical to understand reaction mechanisms and kinetics of surface processes, to design new and better catalysts, perform clean and benign chemistry and intensify existing processes. In our recent work, by combining experimental data with the theoretical insights from our computational colleagues we have been able to explain the structure-activity relationship for a range of reactions of high industrial importance.
Adding Value to Fatty acids and Vegetable oils

Design of New Heterogeneous Catalysts for Selective Chemical Processes
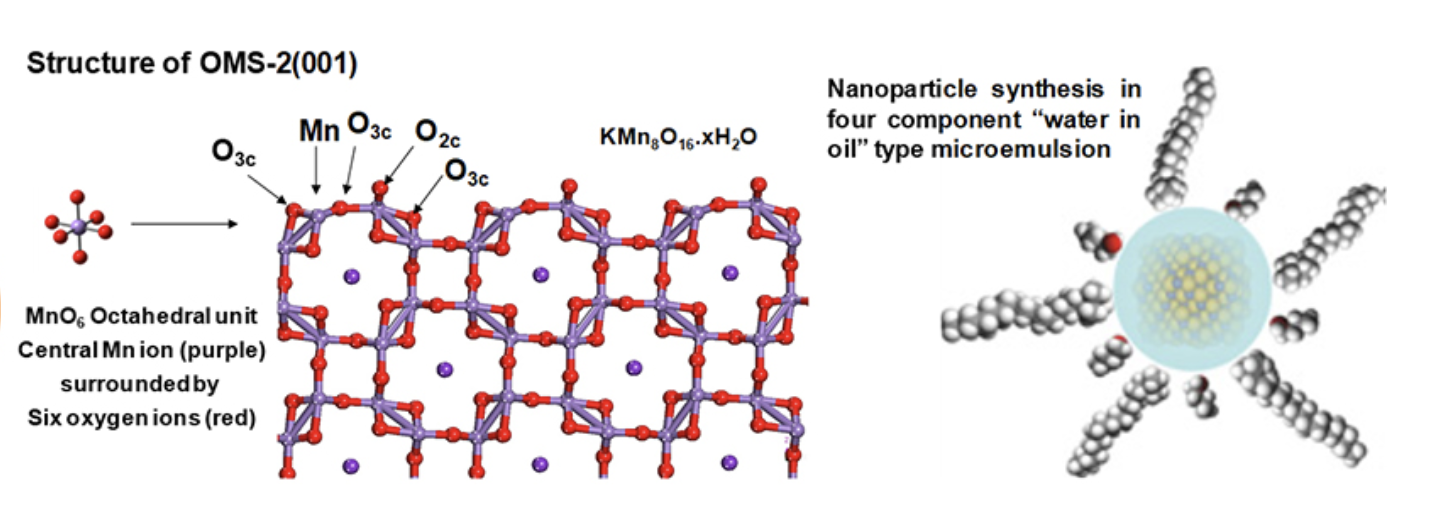
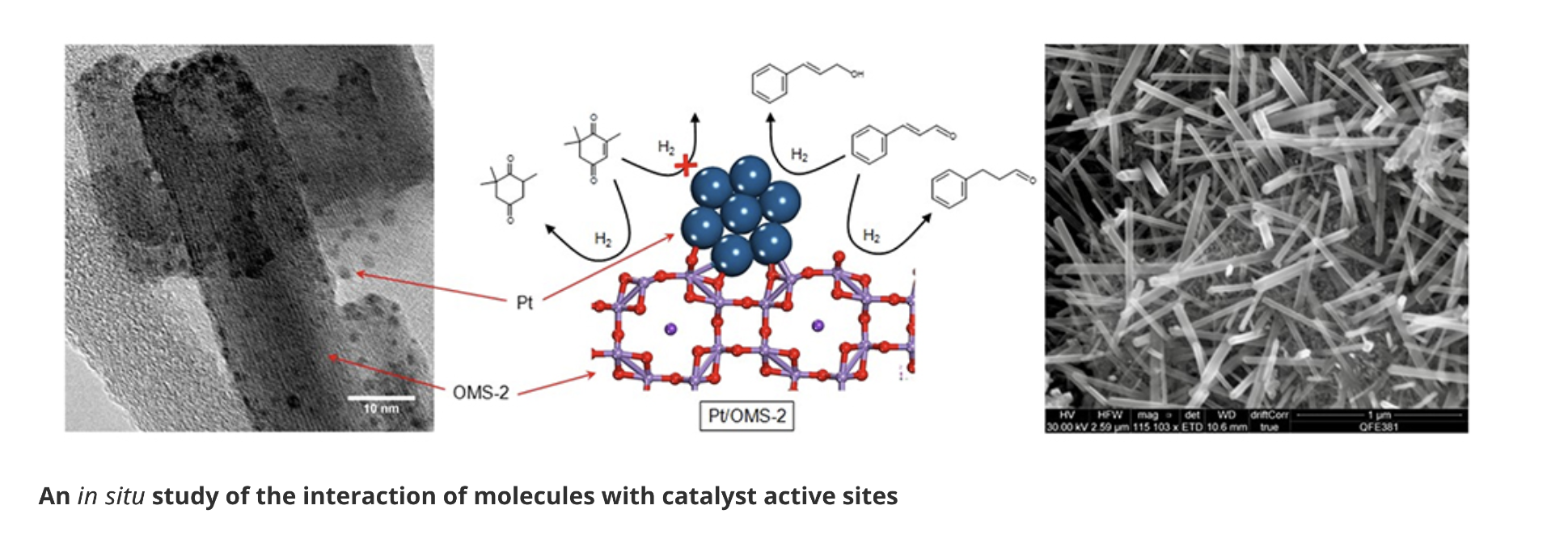
Selective hydrogenation of α,β-unsaturated aldehydes and ketones at the C=C double bond or C=O carbonyl group is highly desirable in the chemical industry. We have achieved highly selective (98% selectivity) hydrogenation using manganese oxide octahedral molecular sieves like cryptomelane (OMS-2) and platinum supported on OMS-2 catalysts at 100% conversions. Density functional theory (DFT) calculations showed the dissociation of H2 on OMS-2 was water assisted and occurred on the surface Mn of OMS-2(001) modified by an adsorbed H2O molecule.
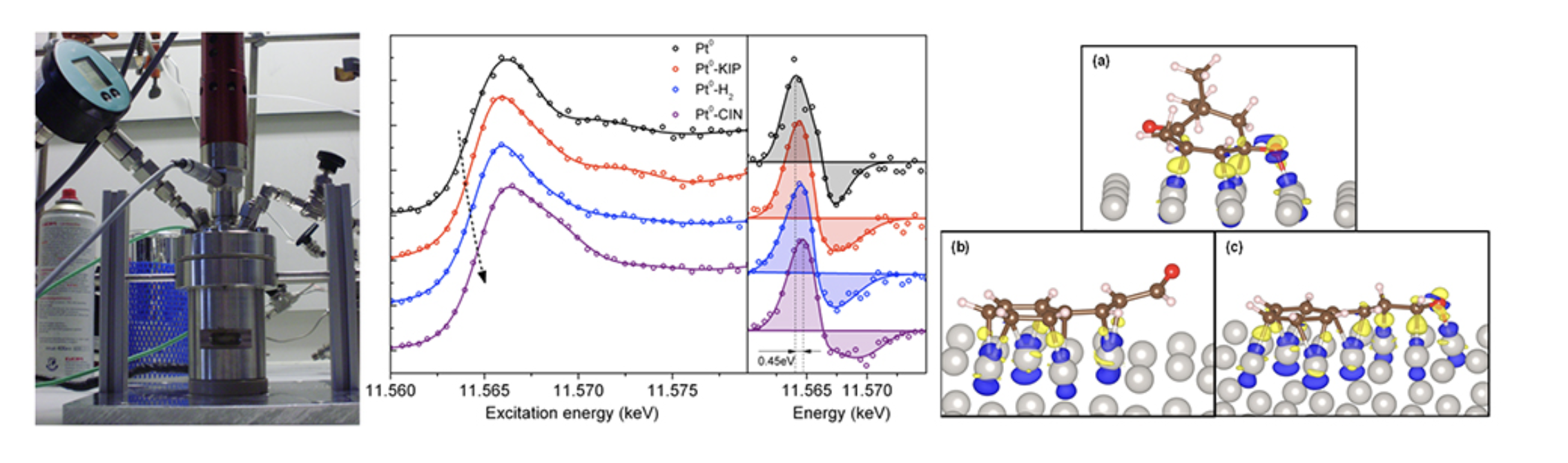
In most of the catalytic chemical reactions, the choice of the catalyst (active catalytic species and support) significantly influences the reaction rate and product selectivity which is the result of the interaction of organic molecules with the active catalyst site, such as adsorption configuration, strength and its influence on the metal electronic structure. We study the adsorption of molecules and its effect on the electronic structure of active metal site by combining the catalytic experiments and Temperature-programmed Desorption technique with an in situ liquid phase X-ray Absorption Spectroscopy and Density Functional Theory calculations. The change in the Pt electronic structure following the interaction of an α,β-unsaturated aldehyde and ketone is shown below. The adsorption of such molecules resulted in an energy shift of Pt Fermi level, which is in good agreement with the molecule adsorption energies calculated by DFT.